Quality Assurance
Overseas Quality Assurance ActivitiesTowards an open system for quality assurance
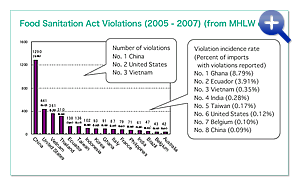
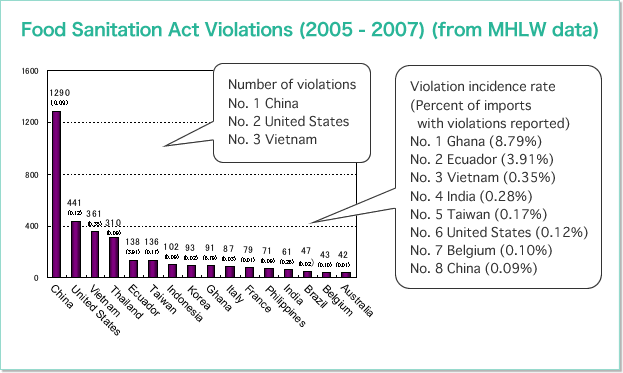
Overseas Quality Assurance Activities
Our concept is The Made in Japan System
- Share Japanese quality standards with overseas partner companies through regular quality inspections
- Firmly establish a Japanese-style quality assurance system
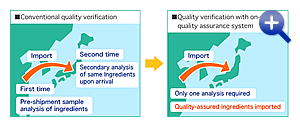
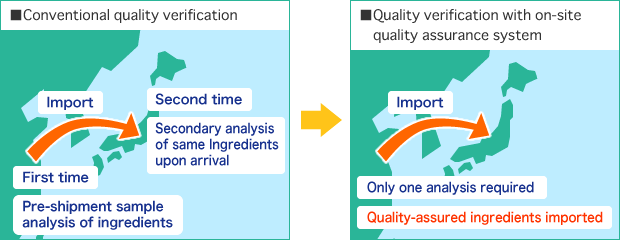
- Conduct thorough on-site quality assurance on upstream ingredients
Step OneShift from manufacturer inspections
- Thorough exchange of analytical techniques
- Thorough implementation of Japanese-style GMP training

Step TwoPromote on-site quality assurance
- On-site sampling
- On-site analysis / analytical precision
- On-site sealing (“food lock”)
Step ThreeFurther promote on-site quality assurance
-
Adoption of API-QMOS (API’s own Quality Management Open System)
- 1. Work improvement activities
- 2. Safety control
- 3. Process control
- 4. Risk management



- Case 1: Long-standing relationship of trust with our partner company
- API-QMOS is an open quality management system of production control and quality control information born from a long-standing relationship of trust.
- Case 2: As a process control initiative
- Allows for genuine sharing of current production control and quality control outcomes.
API-QMOS is effective as a tool to engage in joint deliberation and handling when abnormal situations arise. - Case 3: In work improvement activities
- In the off chance of a claim, API-QMOS is an effective tool for investigating causes, taking corrective action and confirming and assessing outcomes.
- Case 4: In risk management
- API-QMOS is installed on key lines from ingredient preparation and processing through packaging—where risks can be assumed. It is an effective trouble prevention mechanism.
- Case 5: Quality Meetings using API-QMOS
- Allows for sharing of quality standards at regular monthly meetings for work activities, safety and process control.
- Case 6: Strengthening of stringent upstream ingredient control
- API-QMOS is used to strengthen stringent upstream ingredient control and give customers peace of mind through transparency.
- Case 7: Future developments
- Utilization of API-QMOS for lateral expansion (i.e., fully-integrated management) into upstream ingredients and mid-stream control (for manufactured goods) and expansion to other suppliers.